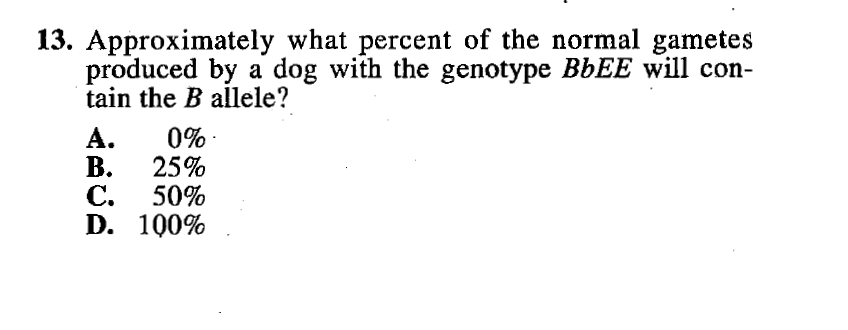ACT Science Prep: Scientific Info to Know
The ACT science section can be intimidating for many students. The biggest reason for this is because it really isn’t all that similar to the science classes students take in high school. In fact, the ACT science section tests students primarily on their ability to analyze and interpret data from various charts, graphs, and texts and requires little to know outside scientific knowledge from the student. However, there are some concepts that it could come in handy to be familiar with on test day. Here, we’re walking you through what types of scientific facts and concepts that you should know before you sit down for the ACT science test because they just might help you reach you goal ACT score.
The ACT science section can be intimidating and frustrating. This is true for a couple of reasons. For one, this section always shows up LAST. You've just spent three hours going through English, math, and reading, and your brain is probably exhausted already, and now you expect it to successfully interpret complex scientific data? (NOTE: Starting September 2020 students will have the option to retake only certain sections of the ACT. Read more about 2020 ACT Changes here.)
Additionally, the science time constraint is pretty intense: 40 questions in 35 minutes (it’s the same time constraint as the ACT reading section). That means you have more questions than you have minutes. The science section is composed of 6 separate studies, each with approximately 5-6 questions associated with it. If you already have a solid grasp of how to analyze and interpret data and can consistently work through the science section studies and answer most questions correctly, then it’s probably time to assess whether your testing pace on this section is appropriate for the intense time constraint.
The science section is also not really what you’d expect from a “science” section based on high school science course curriculum. The ACT science section is basically data interpretation and analysis – there is hardly any outside information that is required to do well on this section, which makes it both harder and easier to prepare for. Like the reading section, everything you need is there on the pages in front of you somewhere. It’s just a matter of finding it, understanding it, and applying it.
For this reason, students who do not know what to expect from the science section may be startled when they try to work through the studies, and students who have little experience with data interpretation may become frustrated. But don’t fear – the ACT science section is one that anyone can master with increased practice and familiarity of the types of science studies that show up and the types of questions asked. ACT science prep focuses on increasing familiarity with the format and wording of the studies, frequent practice of the types of questions asked, and, as always, identifying tips, tricks, and strategies that work for an individual student to help with combatting the intense time constraint of the ACT science section.
Our recommended ACT science test prep plan includes working on many practice studies from real previous ACT exams, identifying a broad ACT science strategy to assist with time management and consistency in answering questions correctly, and leveraging any additional ACT tips and tricks that can assist with speed and consistency.
Before you start to think about what tips and tricks you should make sure to remember on test day, make sure you have a solid big-picture strategy in place that’s going to help you do your best on test day. Once you’ve identified what big-picture ACT science strategies work well to help you attempt as many questions as possible and answer them correctly as consistently as possible, it’s time to consider what other tips and tricks could help you boost your ACT science score even higher. We encourage students to use trial-and-error to determine which ACT science tips and tricks work well for you as you work through the ACT science section.
ACT Science outside info
Lets take a minute to talk about some science background info that really could be helpful on test day.
We’ve already noted that you do not have to be a master scientist in any of the areas being tested in order to do well on the ACT science section. However, some background information and knowledge of certain concepts could definitely help you to answer a few more questions correctly.
The ACT science section has students assess studies and information on areas related to biology, physics, chemistry, animal sciences, earth sciences, genetics, and more. While students do not have to have a deep understanding of these areas, some familiarity of the topics and the types of charts and graphs and labels that that are frequently seen in them can certainly help make working through the studies easier. Many students will already have taken classes in biology, physics, and chemistry by the time that they take the ACT, and this can prove to be very helpful. Students will recognize some of the studies and be able to apply them to their own experiences, which can aid in understanding.
But what about actual facts and figures that students should actually memorize for the ACT? Well, it’s not the end of the world if students opt not to memorize anything before taking the science ACT section. In fact, there are only approximately 4-5 questions on each ACT exam that require some kind of outside knowledge in order to get to the correct answer. But if you REALLY want to reach your ACT goal, especially if that goal is somewhere in the 30s, it is really smart to review and study these concepts prior to test day. It could land you a handful of easy points.
Here are some concepts that students should be familiar with that could show up on the ACT science section:
Biology
Cell biology:
Cell biology questions will require students to know different basic parts of cell biology. Question #12 below is a good example of a question that students may see on the ACT. In the introduction to this particular study (Passage II below), students are given information about a specific species of crab and another of sea grass. Obviously, the crab is an animal species, and the sea grass is a plant species.
Question #12 inquires about the differences in cell biology of the crab and the sea grass. Answering question #12 correctly requires students to recall from prior learning that animal cells contain a nucleus and plant cells contain a cell wall.
The Food Chain
Another question that requires outside information is question #11 seen below. This is from the same study about crabs and seagrass, only this time students are tested on their understanding of the food chain. Answering question #11 requires students to understand that the crab would be higher on the food chain, as it eats the grass.
Chemistry
Freezing & Boiling Point
There may be an occasional question that asks about when a substance is likely to freeze or boil, or some other question of this sort. Knowing the freezing and boiling points of water in both Celsius and Fahrenheit (below) will help make those questions super easy on test day.
Freezing Point
Celsius: 0 degrees C
Fahrenheit: 32 degrees F
Boiling Point
Celsius: 100 degrees C
Fahrenheit: 212 degrees F
pH scale
Most ACT science sections will contain a study that has something to do with pH values. What exactly is pH? pH values are on a scale of how acidic or basic a substance is.
Oftentimes the ACT science study will not ask students about any outside information as it relates to pH values, but once in a while they will, and it will always be some form of the same information: is this substance acidic, basic, or neutral?
What you need to know for the ACT science pH questions is that a pH of below 7 is acidic, above 7 is basic, and at 7 is neutral.
Consider question #39 below. In order to answer this question correctly, students must first have an understanding of what is considered acidic (pH below 7). This information is not provided within the study, but if the student knows this already then this question becomes much easier.
Charges
Some studies include information about protons, electrons, and neutrons and questions will ask about the charges of the particles. This is another topic that the ACT is not likely to give background information on. It is expected that students should have a fair understanding of the charges of these particles.
What you need to know: Protons hold a positive charge, electrons hold a negative charge, and neutrons have no charge. Like charges repel each other and opposite charges attract each other.
Consider question #29 below. Once again, if students are familiar with these particles and how they interact with each other, this question becomes easy. The ACT once again does not give background information on these particles throughout the study, so answering this question correctly requires prior information on the particle types.
Molecules
There could be an occasional question that requires some kind of information about a specific type of molecule.
Glucose
There have been previous ACT exams that include questions about glucose, where students need to know that glucose is represented by C6 H12 O6.
ATP
A somewhat recent ACT exam included question #13 below. None of the answer options are discussed throughout the science study, so, once again, students need to be familiar with ATP in order to get this question correct. ATP is the “energy-rich molecule” that is referenced by the question. Students will have learned this in a science class at some point, so, if they can recall this information, this, too, becomes a fairly easy question.
Phase changes
We already mentioned the freezing and boiling point of water above, but you should also be familiar with other phases and how a substance can go from one state to another. There could be questions that reference phases such as evaporation, condensation, solid, and liquid. Once again, the ACT will not go into background detail about these phases and cycles.
For example: think about water. When water is frozen, it is in solid form. When water warms up, it becomes a liquid. Finally, when you boil water, it evaporates and becomes a gas (steam).
Question #28 below goes with Passage V below. Question #28 asks about what is happening with the movement of liquid from one location to another. In looking at your answer options and the study description, students would be able to rule out answer options like “condensation” and “evaporation” as long as they are familiar with the concepts.
Physics
Gravity
This may seem like common sense, but you may need to use your knowledge about gravity: it is a downward force that acts on objects. You may also have to apply this knowledge to physics studies that include springs and pulleys, which can counteract gravity.
Density
Formula for Density:
Density = mass/volume
Density rules: temperature drops, what happens to pressure question
This question specifically states “If the temperatures…are raised…and if the object neither expands nor contracts with the change in temperature, will the object more likely sink or remain afloat?” You can use the density formula and your understanding of density to work through question #39 below. Yet again, this is a very difficult question to work through without some outside knowledge of density and its rules.
The density formula is a good start for preparing for density-related questions. However, you also need to know some rules about density. Mainly, you need to know that denser objects sink, and less dense objects float.
Take an easy example: a boat on a lake. The boat only floats because it is LESS dense than the water of the lake.
Now consider a rock that is thrown into the same lake. The rock is MORE dense than the water of the lake, so the bowling ball will SINK. The rock weighs more than the same volume of the lake water, so the rock is more dense.
Increasing the pressure on an object decreases the volume of the object and therefore causes density to increase.
Increasing the temperature of a substance lowers its density because it increases its volume. But lowering temperature on an object when the volume is held constant will cause the pressure to decrease as well.
Volume and temperature are directly correlated: Increasing the volume of an object will increase the temperature, and vice versa.
That considered, check out the chart and question #23 below. This question is basically saying “what will happen to the volume if we decrease the temperature?” Per what we just talked about, we know that if the temperature goes down, the volume will go down as well.
It can sometimes be helpful to think of a real-world situation that you understand and know to be true and can apply to some of the scientific studies. For example, consider what happens to car tires in the winter. If you live in a cold place, you will have to put air in your tires in the winter because, as the temperature goes down, the volume of air in your tires will go down as well. That same principle is exactly what is at work in this less-familiar and more complicated example.
Miscellaneous
Colors
The ACT has been making use lately of some questions that require an understanding of colors, specifically black and white. White is the ABSENCE of color, and black is ALL colors together.
Take a look at question #30 below. What color should the tool used in a scientific study be if the scientists DO NOT want the tool to affect the results of their study? That is basically what this question is asking. The answer? WHITE! The scientists would want the tool to have NO COLOR so that it does not affect their research and their results.
Math
It’s important to note that the ACT science section also sometimes requires students to use basic math skills. Students are not allowed to use a calculator on this section, so any required math should not be more difficult than what it is expected that students will be able to do long-hand on their paper, including addition and subtraction, multiplication and division. If a student feels like the math they are trying to do it much more difficult than mental math or long-hand math should be, they are probably doing something wrong.
Lets take a look at a handful of examples, as there are different types of math that you could be required to complete.
Question #13 below requires an understanding of basic fractions and percentages. Students need to be able to figure out that 1 out of 4 is the same as 25% percent and 2 out of 4 is 50%. Pretty basic stuff.
Question #4 below requires basic subtraction. Students are given the boiling temperature of the two substances and must use basic subtraction skills to arrive at the difference.
Note your answer options: they are all fairly far apart. That means that if you are unable to complete perfect math on this question, as long as you can arrive in the ballpark of the right answer, you should be able to make an educated guess on this one. (This is in direct contrast to the ACT math section, in which all wrong answers are answers that students could arrive at if working through a question incorrectly. However, the science section is not meant to test students’ math so much, which is why a calculator is not permitted.)
Question #26 below requires an understanding of the metric system and converting values.
Similar questions could ask students to convert 4000 grams to kilograms or 80 liters to milliliters. It is a wise idea to review the chart below and the different prefixes that denote thousands, hundreds, etc.
We hope you find this helpful and strongly encourage you to review these areas before you take your next ACT test. While you don’t need to spend a ton of time reviewing and memorizing these concepts, taking a little time to do so just might earn you three or four points toward your ACT science goal!